Which Best Describes the Transition From Gas to Liquid
The process involves the ice melting to water the water heating from 0 C to 100 C then the water boiling to steam. Which of these best describes vaporization.
Condensation occurs when a gas is cooled at which time the gas turns into.
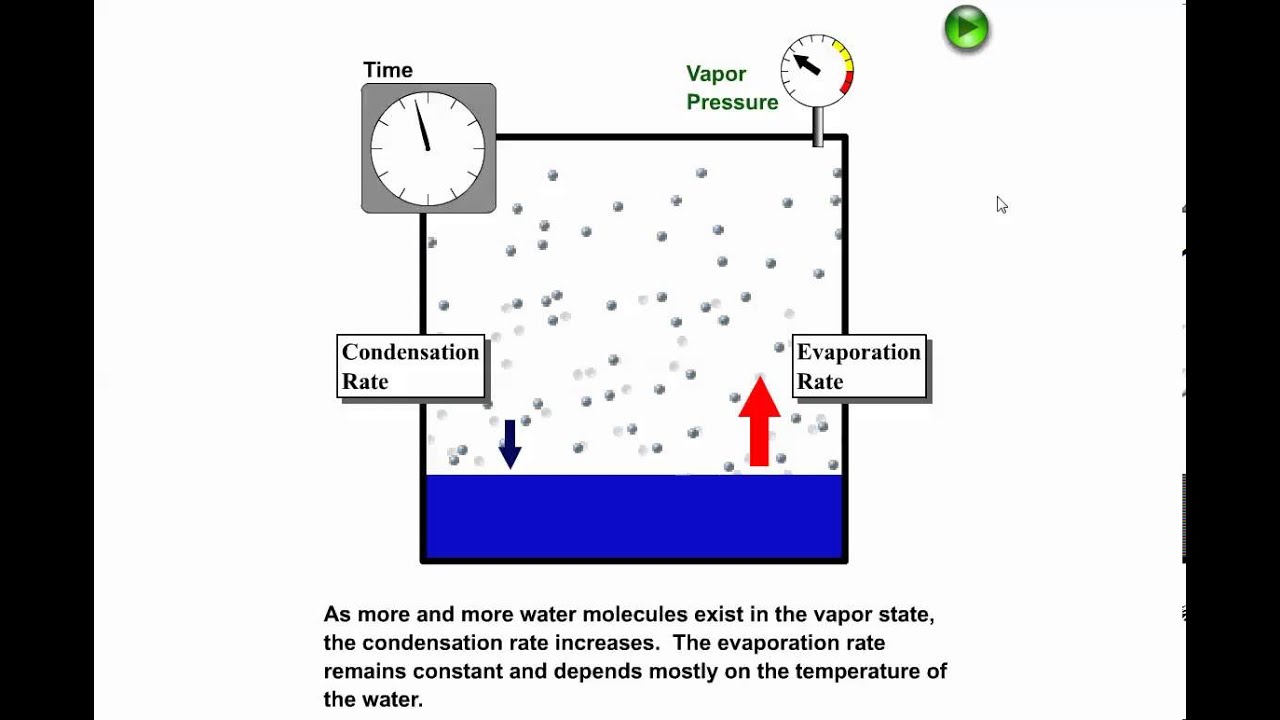
. Gas is higher energy state than liq. The substance moves from solid to liquid to gas form. Lower in density d.
The transition directly from solid phase to vapor phase. 500 x 103 J. Physical changes occur when the form of a chemical substance changes but its chemical composition remains intact.
By signing up youll get thousands of step-by-step solutions to your homework questions. Solid melting liquid vaporization gas. Energy is either removed or added depending on the type of particles.
A liquid is changing into a gas because pressure in the system is increasing. Which best describes the energy change that takes place during. The process by which a solid changes to a gas c.
The energy is usually in the form of heat. There are two types of vaporization. Notice that all the equilibrium conditions involve only intensive quantities.
The specific heat of water is 418 JgC. Sublimation- It is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. Liquid g gas 54 where g GN is the Gibbs free energy per particle.
Energy must be removed because particles in liquid move more slowly. Matter undergoes phase changes or phase transitions from one state of matter to another. Which of the following best describes what is happening in the diagram.
How much energy was released in joules. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. When matter changes from one state to another it is called a phase transition.
The process by which a liquid changes to a gas b. This means that if we have a situation where liquid and gas are in equilibrium then we can have any number N liquid of atoms in the liquid state and any number N gas in the gas state. Evaporation is a phase transition from the liquid phase to the gas phase that occurs at temperatures below the boiling point at a given pressure.
Three common states of matter exist. Map showing phase of a substance at different temperatures and pressures diagram with 3 sections solid liquid and gas water has a different phase diagram left line is tilted more to the left because of the hydrogen bonds. For molecules of a liquid to evaporate they must be located near the surface be moving in the proper direction and have sufficient kinetic energy to overcome liquid-phase intermolecular forces.
Condensation- It is the change of the physical state of matter from gas phase into liquid phase. The diagram below represents a phase change. Energy must be added because particles in liquid move more quickly.
The transition from liquid phase to vapor phase. Which best describes the transition from gas to liquid. However plasma also is a state of matter so a complete list requires all eight total phase changes.
When a material or substance cools and goes from a gas state to a liquid state this process is known as condensation. So ans is a. Which best describes the particles of a liquid compared to those of a gas.
Below is a complete list of the names of these phase changes. This process is useful in separating a solute and solvent from its solution. The pressure exerted by the gas in equilibrium with a pure liquid at a given temperature.
The phase transition is solid to gas so energy will be absorbed. Matter however can change its state with the addition or subtraction of energy. Solids liquids and gases.
The most commonly known phase changes are those six between solids liquids and gasses. So the transition must remove energy. The process by which a substance changes from the solid phase to the liquid phase is known as melting.
Energy must be removed because particles in liquid move more slowly. Condensation is the name of the process through which gas turns into a liquid. Which best describes the transition from gas to liquid.
For molecules of a liquid to evaporate they must be located near the surface be moving. The transition from solid phase to liquid phase. P T and µ.
Gas- liquid bliquid- gas ccannot be determined d. Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase. Five Changes of State are.
CEnergy is neither added nor removed in this phase transition. Sublimation is an endothermic phase transition that occurs at temperatures and pressures below a substances triple point in its phase diagram. The energy absorbed will be the heat of fusion the change in temperature of the liquid the heat of vaporization q fusion q heating q vaporization.
Condensation is the process by which the physical state of a substance changes from its gas phase to the liquid phase. Gas to solid phase transitions are known as depositionGas to liquid phase transitions are known as condensationLiquid to gas phase transitions are known as vaporizationLiquid to solid phase transitions are known as freezing. Describe the transition from gas to liquid.
A gas is changing into a. The process by which a substance changes from the. Which best describes the transition from gas to liquid.
Evaporation occurs at temperatures below the boiling point and occurs on the liquids surface. In an experiment a scientist burned 100 g of methane and the temperature of the 5000 mL of water in the calorimeter changed from 210C to 266C. It can also be defined as the transition of water vapour into water droplets upon contacting a solid surface.
Phase changes are one type of physical change.
What Is It Called When A Gas Transforms Into A Liquid Quora
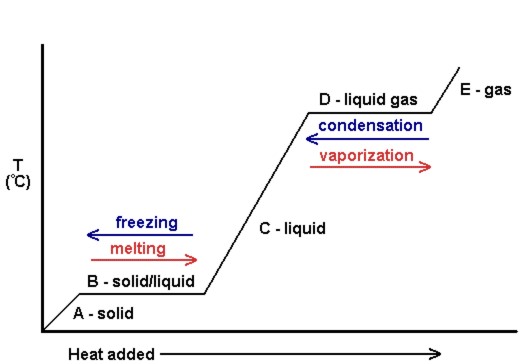
Phase Transitions Melting Boiling And Subliming Introductory Chemistry 1st Canadian Edition

No comments for "Which Best Describes the Transition From Gas to Liquid"
Post a Comment